
Lather was serious business in my house growing up. My dad, a chemist and inventor, specialized in Class A firefighting foams, as well as a special effects “Snofoam” for movie sets, a substance that was the consistency of shaving cream but looked like snow.
We’d do all sorts of experiments in our front yard (we were THAT house on the block), such as measuring how long the foam would last, how high it could build up, whether it would stick to vertical surfaces, and whether it left any residues. I still remember the time he was comparing his foam to a competing product and made a giant “S” with his in our front lawn and a giant “P” with the competitor’s. We had that P burned into our grass for months!
Then there was the promotional picture he wanted for the Snofoam, so I donned my winter parka in June and the one glove I could find (I put my other hand in my pocket) and “looked cold” in the falling foam despite the 90-degree temps.

All this to say, I have many fond childhood memories of foam in its many forms. While helping my dad was simple fun at the time, now that the focus of my work is soap and cleaning, I want to take a deeper look at the science behind the lather. What is its structure, composition, and purpose? What is it good for, and what are its limitations?
What is lather
Knowing what lather can and can’t do begins with understanding what lather is. Whether you’re cleaning your body or your house, lather is what you get when you agitate soap or detergent with water and air to build a matrix of thousands of tiny bubbles. Therefore, in order to understand the structure of lather, we must first understand the structure of bubbles.
When it comes to soap, the simplest bubbles are little air pockets trapped in a spherical film sandwich of soap-water-soap. Picture thousands of these air pocket/soap sandwiches all smashed up together, and you have lather.
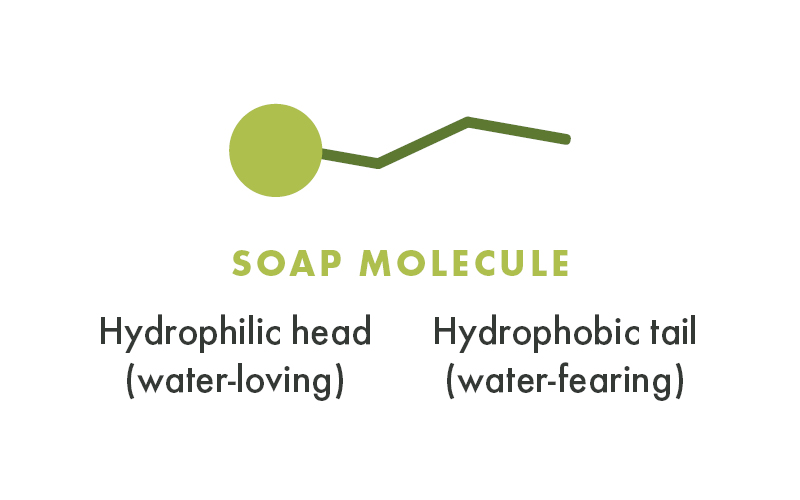
When I say “soap,” that soap can be either a true soap, like Dr. Bronner’s Pure-Castile Magic Soap, or a detergent that might be in the form of a shower gel, bubble bath, shampoo, dish soap, laundry detergent, or the like. The key is that these molecules, shaped rather like a pin, have a rare ability to be attracted to water at their head-end and repelled by water at their tail-end. Not many molecules in nature have this duality.
What the soap molecule is made from—plant oils in the case of the Castile Magic Soap, animal fats with other soaps, or various synthetics in the case of detergents—determines certain characteristics of these bubbles. For example, certain raw materials form larger bubbles and thus a frothier lather, while others make smaller bubbles and result in a creamier lather. The base material also impacts how durable or long-lasting the lather is.
How lather forms
Going back to the individual bubble, the soap-water-soap sandwich which makes the sphere is formed because water molecules love to hold on to each other. Soap molecules break up this party by reducing this ability to a certain extent, partially because they are also attracted to water. The result is the water forms a film, coated on both sides with the water-loving head ends of soap molecules. A beautiful bubble.
When we agitate soap and water by rubbing our hands together, or scrubbing a washcloth over our body, or scouring a brush over a dish, we tumble those soap and water molecules together with some air. The water is doing its best to hold on to itself at the same time as the soap is doing its best to hold on to the water, and so the soap-water-soap trio gets wrapped around little bits of air. This happens many, many times and ends up forming a matrix of thousands of tiny bubbles that we call lather or foam or suds or froth.
Why lather doesn’t do the cleaning
The structure of a bubble is almost the exact inverse of the structure of a micelle, the primary mechanism by which soap cleans. While both are spheres, they differ in four key ways: what is at their center, what is on their outside, their size, and the role of the soap molecules in the structure. At the center of a micelle is oil/grime/gunk. At the center of a bubble is air. The outermost layer of a micelle is composed of the water-attracting heads of the soap molecules. The outermost layer of a bubble is composed of the water-repelling tails of the soap molecule. Micelles are microscopic, measured in nanometers, while the largest bubble on record, was over 6 meters in diameter. However, it is in the role the soap molecules play in each that gets at the crux of the matter. In a micelle, the soap molecules are acting as bridges, linking the oil to water. In a bubble, the soap molecules are acting as insulators, keeping the layer of water from evaporating into thin air.

Soap molecules have a choice. They can either form micelles around oil/grime/gunk OR they can form bubbles around air—but they cannot do both. With the forces of chemistry, they are more drawn to micelle formation if there is any grime present. If there are then excess soap molecules available beyond what is needed to grab hold of grime, there also is likely to be lather present. The key point is that the lather structure is not doing the cleaning. It does not have that ability.
There are detergent cleansers that don’t lather
While lather is usually a benign presence, in some situations, it actually gets in the way of cleaning. Therefore, there are a class of detergent cleansers that are designed not to lather at all. This is the case in automatic dishwashers. (This is one of the reasons that we don’t recommend Dr. Bronner’s Sal Suds or Castile Magic Soap for use in the dishwasher. They lather too much.) Dishwashers aren’t designed to handle bubbles. Bubbly detergents might void the warranty or even break the appliance, and you might end up with a big bubbly mess on your kitchen floor. Fun for the kids, but not for you. Dishwasher detergents certainly clean, but they are designed to do so with no lather.
There are lathering substances that don’t clean
Conversely, there are substances that produce lather, but they aren’t cleaners. The two types of foam my dad worked with are great examples of lathering substances that aren’t designed to clean. The structure of the lather was still that same bubble matrix, but these didn’t need to form micelles and clean. It was the foam structure itself that was key to their performance.
For the firefighting foams, they had a tremendous advantage over straight water in the fighting of fires because they could cling to vertical surfaces, didn’t evaporate as readily as water, could be strategically applied, and especially when dyed bright pink, could be easily seen to tell where they’d been applied and where they hadn’t.
When it came to the Snofoam, the foam made a better snow than either actual snow, which is hard to produce and doesn’t last in warm SoCal weather, or plastic flakes used as snow, which posed a tremendous environmental nightmare when it came to clean up.
Snofoam became the basis of the Magic Foam Experience (MFE), which is part of the community engagement outreach at Dr. Bronner’s. While sometimes for mud runs the MFE team will use actual soap, the original Snofoam is used at schools and other good fun events.
Neither the firefighting foam nor the Snofoam were designed to clean, but both were really good at lathering.
The benefits and purposes of lather
The lather that soaps and detergents produce has a number of uses, even if cleansing isn’t one of them. By no means am I anti-lather, as I hope this list demonstrates.
Lather is luxurious
Let’s not discount the benefit of enjoyment. There is nothing wrong with having things in our lives that simply feel good. We need these. They make us relax, make us smile, make us happy, release some endorphins. The fun of foam also incentivizes spending a few more moments in handwashing–I’d say especially amongst children, but who am I kidding? I like foam, too.
Lather provides lubrication
Lather is functionally a blanket of air, which provides helpful lubrication in shaving. This is a very good thing for keeping skin healthy. Lather helps razors glide smoothly over skin, reducing nicks or razor burn.
This lubrication can also help in washing hair, helping to spread the soap or shampoo through the hair, which helps us use less product and wash more efficiently.
Lather shows where the soap is
For the visually-cued among us, myself included, it is very helpful that foam shows us where we’ve washed and where we haven’t. This helps us to wash more thoroughly. Whether we’re cleaning our hands, our bodies, or maybe even our dog, it helps to see what parts have been soaped up and what parts haven’t.
Lather is reassuring
And this is the crux of the matter. Lather got linked with things getting clean because many soaps do, incidentally, lather. So when we see lather, even though it’s not doing the cleaning, our subconscious says, “Look how clean everything is getting!” Even after reading, and hopefully believing, what I’m telling you here, you’ll likely feel that your cleansing is more effective when there is lather.
Many soap and detergent manufacturers capitalize on this lather=cleaning association by adding filler ingredients to their products whose sole purpose is to lather. They don’t add to the cleaning action and they might even be detrimental to skin health, but they certainly convey a cleaning message.
When Dr. Bronner’s launched its All-One Toothpaste in 2015, we had a lot of conversations, and still do, about the fact that it isn’t nearly as foamy as toothpastes with synthetic surfactants or artificial foaming agents. Without lather, is it even working? Yes, it certainly is, but the lack of lather is a confidence hurdle for many. The absence of synthetic surfactants is a great thing for mouth health because many of them found in common toothpastes are irritating to gums. One trick a customer passed on to me for those who just really need the reassurance of foam is to add a drop of the Castile Magic Soap to their toothbrush along with the toothpaste. This is a perfectly safe thing to do and causes a lot more foam production.
What impacts the amount of lather
There are a number of factors that can increase or decrease the amount of lather you see in a situation.
Soft water makes true soap lather more
Soap lathers more in soft water because the minerals that make water hard—calcium and magnesium—interfere with true soap’s ability to form bubbles. The soap molecules get distracted by their attraction to those minerals and aren’t as available for forming bubbles. With soft water, calcium and magnesium aren’t present to offer this distraction and therefore the soap is free to bubble away.
Detergents lather equally well in hard or soft water
Detergents are immune to the calcium and magnesium in hard water, and so they will produce the same amount of lather in hard water as in soft. This is one reason why detergents are more popular on the market than true soaps. People really like their lather, and since over 80% of the U.S. has hard tap water, folks get their bubble needs met more easily with detergents.
Different base ingredients produce different lather characteristics
When it comes to true soaps in personal care, there is an age-old art to composing the perfect blend of form and function, of cleansing and lathering, from the variety of base ingredient options. Each oil or fat has its own lathering personality and skin impact. As a case study, let’s look at the five oil blend in Dr. Bronner’s Magic Soap: coconut, palm kernel, olive, hemp seed, and jojoba. The first two oils bring the fun to the party. They have a similar fatty acid profile, dominated by lauric acid. Once saponified (i.e. turned into soap), they let loose with abundant frothy bubbles. The latter three oils bring a sedate depth to the mixture, packed as they are with skin soothing nourishment that you can feel in the rich, creamy lather they produce. The lather of the laurics lighten things up while the olive/hemp/jojoba makes sure the work is still getting done.
Summing up the whole foamy story
Lather is about joy, fun, and subconscious satisfaction, which are entirely valid motivations. My purpose here is not to discount the value of lather, but rather to clarify what is happening down in the suds. The bottom line is, you don’t need lather in order to achieve clean, though lather is a delightful perk.



Great read. I remember here and there certain things I had read about lather and why it wasn’t necessary to have within soaps/detergents but never understood WHY. Also, as of late, ‘micellar’ is being used as a buzz word. Almost thought it was fake until reading. Thanks for the morning knowledge!!