
Ever wondered how soap works? Soap is magic. The more I learn about soap, the more it amazes me. Soap is an old substance. Yet with all of our scientific advancements, we can’t make it better. It stands as the first line of protection against every bacteria, every virus, every fungus that seeks to do us harm.
With all that we understand about germ theory and antibiotics and sanitizing, we can’t do better than what the Babylonians (likely) came up with 5000 years ago.
Of course, we know more than they did about why soap works and how best to use it, but study after study backs the power of soap.
What is this miracle worker?
Let me take you down to the molecular level. Most substances either dissolve in water or they dissolve in oil. Soap does both. This is because on a soap molecule, one end is water-soluble and the other is oil-soluble.
Yes! Be astounded!
Let me say it a couple different ways, which all get us to the same place:
One end is water-soluble, the other is oil-soluble.
One end is Hydrophilic and the other is Hydrophobic.
- Hydrophilic – Greek for “water loving,” meaning it is attracted to water
- Hydrophobic – Greek for “water fearing,” meaning it repels and is repelled by water.
One end is Hydrophilic and the other is Lipidphilic.
- Lipidphilic – Greek for “oil loving.” I like this statement; there’s no hate, only love.
One end is Polar and the other end is Nonpolar.
- Polar – has a charge. Water is polar, and since like dissolves like, polar dissolves polar.
- Nonpolar – has no charge.Oils are nonpolar, and nonpolar dissolves nonpolar.
All this adds up to one thing: soap dissolves into both water and oil. Honestly, this stuff gets me terribly excited.

One more bit of Greek and then I’ll stick with English. The soap molecule as a whole is classified as an Amphiphilic molecule. In Greek this means “loves both.” If only we all were amphiphilic.
Do you get what this means? With one end dissolved in water and one end dissolved in oil, soap binds oil to water. It connects them, mixes them and not even if you turn your back on them will they unmix.
This is the equivalent of achieving world peace! There is so much we can learn from the example of soap.
Where does soap come from?
Soap is made by a beautifully efficient one-step chemical reaction. Two inputs. Three outputs. All of the results are useful. No waste. No unwanted leftovers. Beautiful.
Input 1: an animal or plant fat
Input 2: a strong alkali
An animal or plant fat can be tallow (rendered beef fat), lanolin (rendered sheep fat), olive oil, coconut oil, palm oil, palm kernel oil, avocado oil… really any animal or plant fat. My brother David even once used butter for a science fair project. (I don’t recommend making soap out of butter.)
The strong alkali is usually sodium hydroxide – aka lye, aka NaOH – or potassium hydroxide – aka KOH. The former produces solid bar soap. The latter produces liquid soap.
Taking it again down to the molecular level, picture a fat or oil molecule as a big capital “E.” The vertical post is a glycerin molecule, and the three horizontal bars are fatty acids. This earns them the name “triglycerides” – “tri” for the three fatty acids and “glyceride” for the glycerin backbone.
When you combine the oil with the super strong alkali, the ensuing reaction blasts the fatty acids off the glycerin and divides the sodium or potassium ion from its hydroxide OH–.
All these parts reform:
- The fatty acids bond with the sodium or potassium, forming soap.
- The OH– (hydroxide) ions get together and form H2O – water!
- The glycerin decides it likes the solo life and hangs out by itself.
Two inputs. Three outputs. Oil and alkali in. Soap, water, and glycerin out. It’s just so tidy.
Soap in action: surfactant & emulsifier
Before soap gets to its peacemaking action mentioned above, it has to prepare the water. Water is an exclusive substance. It’s rather clique-ish. All it wants is to hang out with itself. The scientific term for this snobbery is surface tension.
Surface tension is the cohesive force a liquid has between molecules of its kind. With water, surface tension is pretty high, so much so that it somewhat resembles an elastic membrane. Surface tension is why water beads up on a glass surface, how some bugs can walk on water, and why belly flops are so very painful.
Water’s surface tension also makes it skim over surfaces instead of penetrating down into them. Pure water doesn’t make things totally wet. You may be thinking, “I’m pretty sure water makes me wet. It doesn’t skim over me.” True you get wet, but not as wet as you could.
Soap breaks this surface tension of water, forcing the water molecules to let go of each other, to look around and see that there are other things worth exploring. Things like fibers and pores and other microscopic crevices in surfaces that need to be cleaned. Soap makes water wetter.
When soap breaks the surface tension of water, it allows the water to penetrate more fully down to the surfaces that need to be cleaned, whether it’s a fabric, a counter, hair, or your skin. This ability makes soap a “surfactant” – which is a snazzy little mashed up word drawn from the phrase, “Surface Active Agent.”
Then, once the water with the assistance of soap has gotten itself down to the surface in need of cleaning, soap then gets to work with its great peacemaking mission I described above.
Soap’s ability to bond oil and water has a scientific name: emulsifying. Soap is an emulsifier. But it doesn’t do this emulsifying on a one-to-one, one soap molecule for every oil molecule basis. Instead, it acts in large groups.
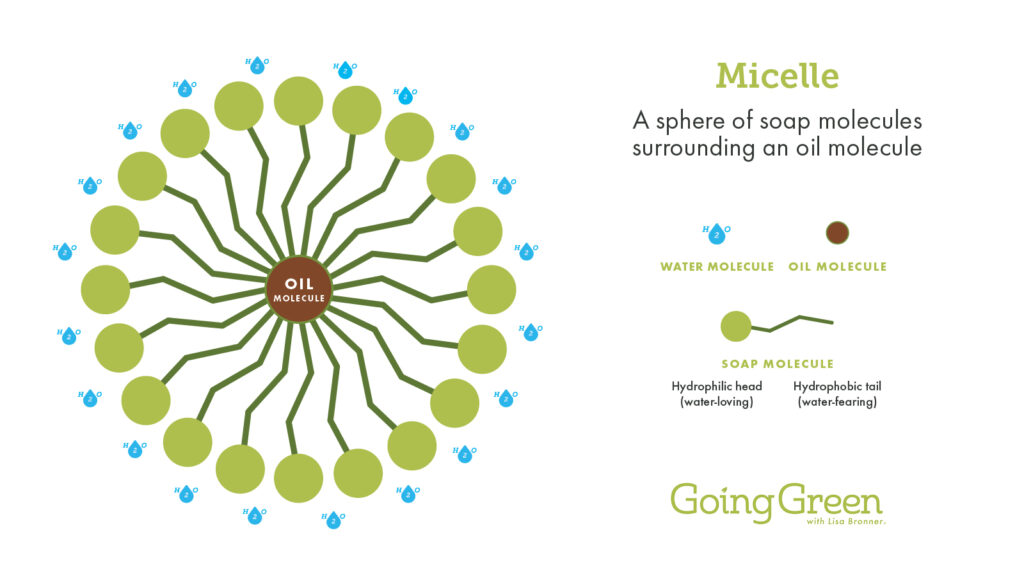
A whole bunch of soap molecules – around 60 – will surround each bit of oil, forming a sphere around it, tucking the oil away safely in the middle. This little oil/soap nugget is called a micelle. It takes some time and jostling for soap to get itself situated in this way, which is why there’s the recommendation for 20 seconds of handwashing.
The surface of the fully formed micelle consists solely of those water-loving soap ends, which is all the surrounding water senses. The water has no idea that its archenemy oil is hiding inside. As the water passes by, it picks up those micelles by all those hydrophilic heads and carries them away. This is how soap cleans.
But what about germs!
All along here, I’ve been talking about soap’s action against oils. But it’s not only oils on my hands that concern me. A bigger concern is germs – bacteria, viruses, and such. Happily for us, germs are coated in a lipid layer. Remember lipid means fat. So as far as the soap can sense, bacteria and viruses are oil. Soap attaches to them the same way it would to a bit of olive oil. Its oil-soluble end dissolves right into that lipid layer, tucking it into a micelle, and swishing it away. Good-bye, germs. Hello, health.
What about Antibacterial Agents?
In recent decades soap had a challenger to its cleaning supremacy: Antibacterial Cleansers. Novel antibacterial agents seemed to elevate the effectiveness of hand and surface cleaning. These seemed like they might keep us even safer than soap ever could.
However, after a couple decades of dominance, where humble soap appeared to be bested, research caught up with the marketing. The Food & Drug Administration (FDA) demanded proof from manufacturers that antibacterial soaps were indeed more effective than regular soap, as marketing and labeling implied. Despite the huge market share that was at stake, no convincing research was found. Further, antibacterial agents give people a false sense of security and may possibly contribute to the rise of antibiotic resistant bacteria. The FDA banned the sale of antibacterial hand and body cleansers containing any of 19 active antibacterial agents, including the ubiquitous Triclosan. They reaffirmed their stance that soap is all you need.
In Summary:
- Soap is a surfactant and an emulsifier.
- Soap works primarily by removing, not by killing, though some germs are killed in this process.
- Soap needs some time and action to get itself situated in all those tidy micelle clusters. Thus, the recommended 20 seconds for handwashing.
- Soap needs to be rinsed with water. It does not work waterlessly.
This is my ode to soap. I think soap is pretty great. I hope knowing how it works gives you more confidence in it and makes you want to spend a little more time with it.
Further Reading
If you’re tired of this new-fangled digital business and yearn for a tangible book, check out Theodore Gray’s excellent tome Molecules, especially Chapter 4 “Oil and Water.” Soap is the superhero. Don’t just look at the pictures, riveting though they are. Read the words.
Further reading
- What Can You Mix with Dr. Bronner’s Sal Suds?
- What Can You Mix with Castile Soap?
- Sal Suds Dilution Cheat Sheet
- Dilutions Cheat Sheet for Dr. Bronner’s Liquid Castile Soap
This tip and many more are in my book, Soap & Soul: A Practical Guide to Minding Your Home, Your Body, and Your Spirit with Dr. Bronner’s Magic Soaps, available now in hardback on DrBronner.com or at your favorite bookseller, and as an eBook and audiobook (read by me!) from wherever you download or listen.








Hi Lisa,
I have asked this Q a few times about how to add soap cream either by itself to a foam pump, or in addition to liquid foam castile soap in a foam pump.
Is there an answer? If not, I guess I will keep using trial and error. I love how the soap cream feels, but due to some physical limitations, I try to make a batch of something once and have it last a while. I was making soap cream an keeping it in the refrigerator, but it became a bit of a chore for me. Ideally, I would love to make a batch and augment the (super quick and easy) liquid foam soap to make each formula moisturizing enough and also handy to prepare.
I would love your input!
If there is no answer, would you be able to email me and let me know?
I will stop bombarding you with this same question lol!!
Thank you…♥
Hi Age – Thanks so much for your patience. I had to try this out for myself and the results weren’t clear cut. I was able to make a foaming soap out of the soap cream made from bar soap. I diluted the soap cream 1:1 with water. The foam this created was a little squirty rather than a creamy foam, but it did work okay. As you can tell, I’m not completely pleased with the results, but it does work. All the best – Lisa.
Thank you – in my mind, I was wondering if you weren’t answering right away because you were trying it out, but I didn’t want to make up a whole story, lol! I wondered this because I didn’t see any formula anywhere, AND I know you try everything out in a detailed, scientific way. I appreciate your taking the time to experiment.
Since 1:1 isn’t ideal, maybe 1 1/2:1 soap to water might work. Did you by chance also try adding the soap cream to liquid castile soap that was made 4:1 ? When I first tried doing that, I must’ve hit it lucky because the result was perfect. The SC added the right amount of moisturizing effect and was a just a little more luxuriously foamy. The convenience and the end result was worth it. BUT… I haven’t had the same luck since the first couple of times! It’s been either too thick or too thin.
I’m going to keep trying to find the right amount!!
Thank you!!!
Hi Age – It sounds like you’re on the right track with the mixture! I’ll keep fiddling with mine and if I come up with anything worth sharing, I’ll let you know.
Extremely well worded. A beautiful description of the magic-science of soap!
Wonderful update, and awesome breakdown of the differences…
Can I use pure castle soap for shampooing my hair ?
Hi Gwendolyn- I’ve been using Castile Soap to wash my hair for more than 10 years now. The key to using a true soap to wash hair is to use an acidic rinse – either ACV or the Dr. Bronner’s Citrus Hair Rinse. After I have thoroughly rinsed out the soap, I dilute a capful of the Citrus Organic Hair Rinse in a cup and then pour it over my head and work it through and rinse thoroughly. Alternatively, use about 1/2 cup apple cider vinegar diluted with 1/2 cup water as an acidic rinse. During the couple weeks of transition, especially if you’re coming from a conventional shampoo/conditioner regimen, you may need to use another crème conditioner after the rinse. This article has more on washing hair with Castile Soap, https://bit.ly/ShampooToSoap
Well, that was very interesting and also very entertaining! I didn’t realize there was so much I didn’t know about soap and also about how it gets rid of germs.
This is very informative. I love it!
Excellent! Thanks, Ron!
I’ve been using Dr. Bronner’s soaps for years!
A microfiber mop and Dr. Bronner’s in a bucket or spray will not only outclean anything else for your floor, but the whole house will smell cleaner! I follow the package directions and I pick up the mop water on the floor with old clean towels. And I have mostly hardwood floors. People compliment me on them often.
Dr. Bronner’s removes makeup and sunscreen (including most eye makeup) in one fell swoop and is non irritating if you keep your eyes closed. Oddly, it doesn’t try out my skin. I use the lavendar bar soap.
I’ve tried a lot of other products, but Dr. Bronner’s bar soap is the only one step product (less friction is better) which is very important for rosacea. If you have hard water you can also splash some witch hazel/aloe toner on your face or use distilled water with a bit of apple cider vinegar added.
I use Dr. Bronner’s castile soap to clean my counter tops and sinks, usually the Citrus, Lavendar or unscented. The funny thing is a new squirt of soap usually cleans off the old soap scum!
If you like to wear organic cotton or wool, Dr. Bronner’s castile works perfectly and everything smells better, not to mention that stain removal is easy with just some extra dabbed on full strength.
There is no better product to wash your bed sheets than Dr. Bronner’s castile lavendar soap. Softer sheet than are cleaner and smell better and all greasy stains will be gone.
Dr. Bronner’s soaps are great to bathe your dog and will kill fleas on the spot! Unfortunately, the effect doesn’t last forever, but it helps to wash the dog bed or cover in Dr. Bronner’s also.
Hi Mary – I love hearing all of these ways you’ve put the Castiles to use! Sounds like you and I have similar households!
I absolutely loved this article! Thank you for writing it and for assuming we’re intelligent beings capable of understanding. I have used Dr. Bronner products for more than 40 years and and will never stop appreciating them and the fine people who produce them. Thank you!
Hi Ron – Ha! Thanks! I do have that assumption about people. I’ll proffer it’s one of the better assumptions to have! Glad you’ve been enjoying our products for so long!
Thank you for your work, this is a great site!
I had no idea soap was magical but then I guess everything about life is, because of all the atoms involved.
I would like to hear what “The Bronner touch” thinks about quantum physics and our world. Yep, it’s another subject I know nothing about.
Thanks again,
Den
Hi Den! Thanks for your kind words! There is certainly a lot going on around us that we cannot see. We happen to have a quantum physicist on staff. He’s no longer working in that field, but I reached out to him with your question. There’s a lot to think about in his response: “The Heisenberg uncertainty principle shows that we cannot precisely know the position and momentum of a particle at the same time. The more that we try and measure one, we change the other. So there are things in the world that we just cannot know all of. Quantum coupling can cause small changes at one location to be reflected as quantum state at a remote location. So what happens here can have effects everywhere and vice versa. I like to think that Schrödinger’s cat is alive until observed otherwise. Through quantum mechanics are are all part of one grand universe. All One!”
This was a fun read! I appreciate the detailed and entertaining explanation. My dad was a chemist and I, being a rebellious kid, took a couple biology classes and a physics class (which I loved!) in college, but I refused to take a chemistry class. Now I wish I had learned about chemistry – especially seeing how practical it can be. 🙂
Ha! Sounds like we have similar roots! My dad was also a chemist, but completely self-trained. Never went to college, barely graduated from high school. So his chemistry was all hands on learning. I did take some chemistry classes, but so preferred my dad’s way of learning. Much more fun!
Love this type of post!! Yes, I love the tips and info about Dr Bronner’s soaps but knowing how they do what they do is really great. Specially during this crazy times with tons of antibacterial soaps and people afraid of the soap bar. Thanks Lisa!!
Is there the dreaded “Soap Scum” left behind with your product? On another note, how do I get rid of soap scum left by bars of soap used in the shower?
Hi Kyle- Any true soap leaves behind soap scum – which is actually a salt produced from a reaction between the soap and minerals in hard water. It’s the downside of a true soap, but pretty easy to remedy. Dissolve it with a spray of half water and half vinegar (don’t do this on marble or other soft stones though), or scour with baking soda. If you have a glass shower door, going over it with a squeegee after showering helps minimize build up there. For more on removing soap scum, see my blog post: https://www.lisabronner.com/scum-scum-go-away/
This was truly informative and interesting!
In fact, I will use soap slightly differently now that I understand how it works, thank you!
Excellent!
Why do I feel like I just took a chemistry class that I finally understood? This made me feel even better about using your soap.
Thanks, Beverly! Glad you found this helpful!
I absolutely love when you do these type of posts!❤🧡💛💚💙💜
Thanks, Nicole! That’s great to hear!